-
PDF
- Split View
-
Views
-
Cite
Cite
Silvia I. Cazorla, Marina N. Matos, Natacha Cerny, Carolina Ramirez, Andrés Sanchez Alberti, Augusto E. Bivona, Celina Morales, Carlos A. Guzmán, Emilio L. Malchiodi, Oral Multicomponent DNA Vaccine Delivered by Attenuated Salmonella Elicited Immunoprotection Against American Trypanosomiasis, The Journal of Infectious Diseases, Volume 211, Issue 5, 1 March 2015, Pages 698–707, https://doi.org/10.1093/infdis/jiu480
Close - Share Icon Share
Abstract
We have reported that attenuated Salmonella (S) carrying plasmids encoding the cysteine protease cruzipain (Cz) protects against Trypanosoma cruzi infection. Here, we determined whether immunoprotection could be improved by the oral coadministration of 3 Salmonella carrying the plasmids that encode the antigens Cz, Tc52, and Tc24. SCz+STc52+STc24–immunized mice presented an increased antibody response against each antigen compared with those in the single antigen–immunized groups, as well as higher trypomastigotes antibody-mediated lyses and cell invasion inhibition compared with controls. SCz+STc52+STc24–immunized and –challenged mice rendered lower parasitemia. Weight loss after infection was detected in all mice except those in the SCz+STc52+STc24 group. Moreover, cardiomyopathy-associated enzyme activity was significantly lower in SCz+STc24+STc52–immunized mice compared with controls. Few or no abnormalities were found in muscle tissues of SCz+STc24+STc52–immunized mice, whereas controls presented with inflammatory foci, necrosis, and amastigote nests. We conclude that a multicomponent approach that targets several invasion and metabolic mechanisms improves protection compared with single-component vaccines.
Trypanosoma cruzi, the causative agent of the neglected tropical disease American trypanosomiasis, or Chagas disease, is an obligatory intracellular parasite that affects 10–12 million people in Latin America. Although vectorborne transmission has been reduced to 70% in Southern Cone countries, mother-to-child transmission, transfusion- and organ transplantation–associated infections, and ingestion of contaminated foods have emerged as important transmission routes [1–5].
In addition, due to regional and global migration caused by economic hardship, the geographic region of Chagas disease has expanded from rural to urban areas and to nonendemic countries in Europe and North America, a phenomenon referred to as the globalization of Chagas disease [6–8]. The treatment of every affected individual is an ethical imperative. Nevertheless, major antiparasitic drugs are toxic, sometimes unavailable, and ineffective during the chronic stage. Current prevention strategies are insufficient for controlling disease, thus a prophylactic vaccine against T. cruzi is urgently needed.
Trypanosoma cruzi contains a major cysteine proteinase called cruzipain (Cz). Protease-deficient parasites rapidly activate host macrophages and cannot survive [9]. We have reported the efficacy of attenuated Salmonella enterica (S) carrying a plasmid encoding Cz (SCz) in protecting against T. cruzi infection [10]. Development of multicomponent vaccines constitutes an attractive strategy for triggering immune response against several target structures, thereby affecting different key processes (eg, invasion, survival, metabolism). In this regard, we selected antigens that play a key role in the parasite life cycle and determined whether immunoprotection elicited by SCz could be improved by coadministration of Salmonella strains carrying plasmids encoding a thiol-transferase (Tc52) and a 24-kDa flagellar calcium-binding protein (FCaBP or Tc24).
Tc52 is a T. cruzi thiol-disulfide oxidoreductase release protein described as the missing link between the reduced glutathione- and trypanothione-based metabolisms [11]. Tc52 exerts regulatory effects on host inflammatory and immune responses [12], and it is considered a virulence factor crucial for parasite survival [13, 14]. Tc24 is a 24-kDa highly immunogenic protein that is present in all developmental stages of the parasite and that plays a key role in flagellar motility, leading to cell invasion by trypomastigotes [15, 16].
Incorporation of any of these 3 antigens in a monocomponent prophylactic or therapeutic vaccine resulted in reduced parasitemia [14, 17–20]. Here, we demonstrate that a multicomponent oral vaccine based on Salmonella as a DNA delivery system for Cz, Tc24, and Tc52 elicited strong humoral and cellular immune responses, conferring improved protection against T. cruzi infection with respect to monovalent formulations.
MATERIALS AND METHODS
Recombinant Proteins and Eukaryotic Expression Plasmids Encoding Cz, Tc52, and Tc24
Recombinant Cz purification as well as the construction of eukaryotic plasmid with 1404 bp encoding the full length Cz was previously described [19]. Tc24 was cloned and expressed similarly. The cloning and expression of Tc52 in prokaryotic and eukaryotic plasmids is described by Matos et al [21].
Immunization and Challenge
Experiments that used animals were approved by the Review Board of Ethics of the Instituto de Estudios de la Inmunidad Humoral and conducted in accordance with the guidelines established by the Consejo Nacional de Investigaciones Científicas y Técnicas
Antibody Determination
Protein-specific antibody titers were determined using enzyme-linked immunosorbent assay (ELISA) in plates sensitized with 0.2 µg of Cz, Tc52, or Tc24 per well. Peroxidase-conjugated goat immunoglobulins to mouse immunoglobulin (Ig) G or biotinylated rat monoclonal antibodies to mouse IgG1 or IgG2a subclasses were used as the secondary antibody, followed by streptavidin-peroxidase when appropriate. Plates were developed by adding o-phenylenediamine (OPD)/H2O2. Antigen-specific intestinal secretory IgA (sIgA) was detected by ELISA using plates coated with anti-IgA antibodies and purified IgA as the standard.
Delayed-type Hypersensitivity Reaction
The delayed-type hypersensitivity (DTH) test was performed 13 days after the last immunization by intradermal challenge with 5 µg of the corresponding protein into the footpad. Results are expressed as the difference in thickness of footpads before and 48 hours after the inoculation [10].
Cell Invasion Inhibition
Vero cells (5 × 103 cells/well) were infected with transfected blood trypomastigotes expressing β-galactosidase [23] at a parasite-to-cell ratio of 10:1 for 24 hours at 37°C. Trypomastigotes were previously incubated in triplicate with diluted (1/50) serum from mice belonging to each immunization group. Controls included uninfected Vero cells pretreated with Roswell Park Memorial Institute 1640 medium alone (0% infection), and cell monolayers were infected with untreated trypomastigotes (100% infection) [24]. After 24 hours of coculture, plates were washed with phosphate-buffered saline and fresh complete medium was added. The assay was developed 5 days later as described [25, 26].
Complement Mediated Killing of Parasites
Trypomastigotes were incubated in triplicate with diluted (1:10) decomplemented sera from mice belonging to each immunization group. Incubation for 30 minutes at 37°C with 5% CO2 was performed in the presence of normal donor fresh serum [24]. Then, the number of living parasites was determined by counting in a Neubauer chamber.
Measurement of Muscle Damage
Muscle injury was evaluated through the determination of a panel of myopathy-linked enzyme markers at 100 days post-infection (dpi). The assays were performed as described [10]. The histological features of heart and skeletal (quadriceps) muscles from vaccinated and infected mice (100 dpi) were also investigated. A blind histological test was performed as previously described [27].
Statistics
Statistical analyses were carried out with the Prism 3.0 Software (GraphPad, San Diego, California) using 1-way analysis of variance. The log-rank test was used for survival curves. P values < .05 were considered significant.
RESULTS
Multicomponent Vaccine Elicits Systemic and Local Humoral Immune Responses
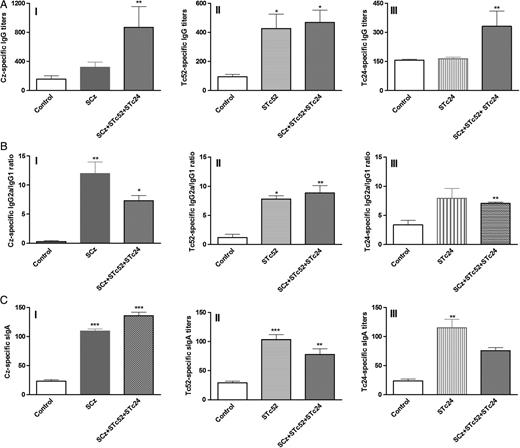
Antigen-specific antibody responses in vaccinated mice. C3H/HeN mice were orally immunized with Salmonella carrying eukaryotic expression vectors encoding cruzipain (SCz), Tc24 (STc24), Tc52 (STc52), or all 3 antigens (SCz+STc24+STc52); control mice received Salmonella transformed with the backbone vector pCDNA 3.1 (+). Two weeks after the last immunization, sera and intestinal lavage from mice were assayed using enzyme-linked immunosorbent assay for the presence of antigen-specific (A) immunoglobulin (Ig) G, (B) IgG1 and IgG2a (shown as a IgG2a-to-IgG1 ratio), and (C) intestinal sIgA, against (I) rCz, (II) rTc52, and (III) rTc24. Antigen-specific sIgA in each sample was normalized to the total IgA present in the lavage. Results are expressed as antigen-specific sIgA titers per microgram of total IgA. Each bar represents the group mean (N = 5)±standard error of the mean. Stars at the top of a bar indicate comparison with the control group. *P < .05; **P < .01; ***P < .001. The results correspond to 1 representative of 3 independent experiments.
Considering that T. cruzi is able to penetrate intact mucosae, the ability of a vaccine candidate to efficiently stimulate both systemic and local immune responses would represent a real asset. Accordingly, we determined whether the vaccination protocols were able to stimulate sIgA in intestinal lavage fluids. Antigen-specific sIgA was detected in all vaccinated animals (Figure 1C). Taken together, these results show that for vaccination using Salmonella as a DNA delivery system for Cz, Tc52, and Tc24 induce antigen-specific antibody responses at mucosal and systemic levels.
Antigen-Specific Antibodies Kill Parasites and Limit T. Cruzi Infection In Vitro
Biological activity of antibodies elicited in vaccinated mice. C3H/HeN mice were immunized with Salmonella carrying vectors encoding cruzipain (SCz), Tc24 (STc24), Tc52 (STc52), all 3 antigens (SCz+STc24+STc52), or pCDNA 3.1 (+) as control. Two weeks after the last immunization, sera were obtained and evaluated for their activity to mediate (A) complement-dependent trypomastigote lysis and (B) in vitro inhibition of trypomastigotes infection of Vero cells. Cell invasion inhibition was calculated as 100 – [(absorbance of treated infected cells)/(absorbance of untreated infected cells) × 100]. The results are representative of 3 independent experiments. The star at the top of a bar indicates that values were statistically different with respect to those obtained using sera from mice in the control group at *P < .05, **P < .01, and ***P < .001.
Cellular Immune Response
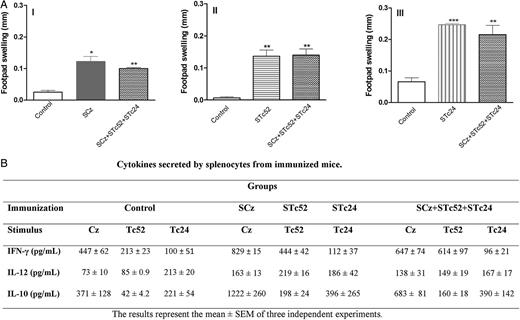
Cellular immune response in immunized mice. A, Delayed type hypersensitivity test. Mice were immunized with Salmonella carrying plasmids encoding cruzipain (SCz), Tc24 (STc24), Tc52 (STc52), all 3 antigens (SCz+STc24+STc52), or pCDNA 3.1 (+) as control. Fourteen days after the last immunization, footpad thickness was measured before and 48 hours after the inoculation of 5 µg of (I) rCz, (II) rTc52, and (III) rTc24. Results are expressed as the differences between the values of measurements performed before and after antigen inoculation. Stars at the top of a bar indicate that values were statistically different with respect to those obtained in the control group at *P < .05, **P < .01, and ***P < .001. B, Cytokine production in immunized mice. Spleen cells were harvested 2 weeks after the last immunization and cultured in duplicate in the presence of every recombinant antigen. Supernatants were collected 48 hours later and assayed in duplicate by capture enzyme-linked immunosorbent assay (BD Bioscience, OptEIA), according to manufacturer's instructions. Abbreviations: IFN-γ, interferon-gamma; IL, interleukin; SEM, standard error of the mean.
To assess the cytokine profiles in immunized mice 15 days after the last boost, we examined the levels of interleukin (IL)-12, interferon-gamma (IFN-γ), and IL-10 in supernatants of splenocytes after in vitro restimulation with rCz, rTc52, and/or rTc24 (10 µg/mL). Cells from SCz- and STc52-immunized mice released higher levels of Th-1–associated cytokines than controls (Figure 3B). In contrast, the level of cytokines produced by cells from STc24-immunized mice was not significantly different with respect to controls. In accordance with these results, when we analyzed cytokine levels in the group receiving the multicomponent vaccine, we observed a strong release of IFN-γ and IL-12 upon Cz and Tc52 in vitro restimulation, whereas no differences were observed upon Tc24 stimulus with respect to controls. In addition to the Th1 cytokines, the immune modulatory cytokine IL-10 was released by cells from the SCz+STc24+STc52 group upon stimulation with every antigen.
Salmonella-based Multicomponent Vaccine Elicited Protection Against T. cruzi Challenge
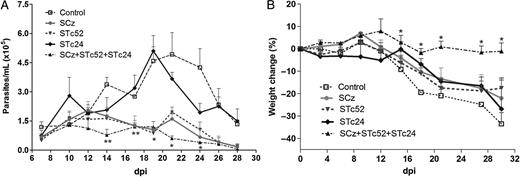
Protection against acute phase of Trypanosomacruzi infection. C3H/HeN mice were immunized with Salmonella carrying plasmids encoding cruzipain (SCz), Tc24 (STc24), Tc52 (STc52), all 3 antigens (SCz+STc24+STc52), or pCDNA 3.1 (+) as control. A, Parasitemia. Fifteen days after the last boost, mice were challenged intraperitoneally with 100 trypomastigotes of the RA strain; the number of parasites was determined in 5 µL of fresh blood. B, Weight change in mice. Results show the difference between the weight on each day and the weight registered the day of the infection (day 0) × 100. All comparisons were referred to the control group. Differences were statistically significant at *P < .05 and **P < .01. Abbreviation: dpi, days post-infection.
As an indirect parameter of the protection conferred by vaccination, we evaluated mice weight as a function of time during the acute phase of infection. Mice immunized with single-component vaccines showed slightly less weight lost than nonvaccinated controls (>30%) following challenge (Figure 4B). In contrast, mice immunized with the multicomponent formulation (SCz+STc24+STc52) maintained their weight until day 30 post-infection (P < .05 with respect to the control group).
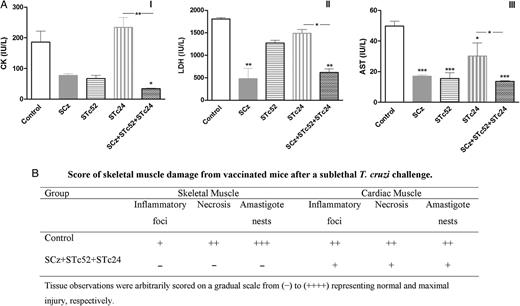
Protection in the chronic phase of Trypanosomacruzi infection. A, Serum levels of myopathy-linked enzyme markers from immunized and infected mice. Blood was collected on day 100 post-infection, and assays were performed to determine the levels of creatinine kinase (CK, I), lactate dehydrogenase (LDH, II), and aspartate transaminase (AST, III). The bars represent the average ± standard error of the mean. The star at the top of a bar indicates that the values were significantly different with respect to the phosphate-buffered saline (PBS) group at *P < .05, **P < .01, and ***P < .001. B, Score of skeletal and cardiac muscle damage from vaccinated mice after 100 days of T. cruzi challenge. The organs were rinsed with PBS and fixed in 10% buffered formalin. Fixed tissues were embedded in paraffin, sectioned, stained with hematoxylin-eosin, and blindly examined by light microscopy.
Comparison Between a Bicomponent and the Multicomponent Vaccines
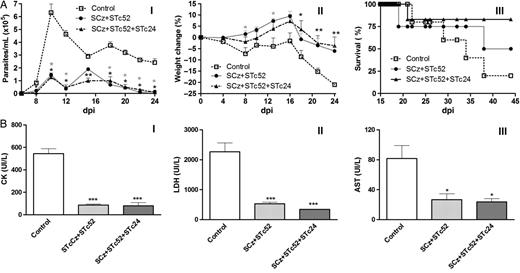
Protection against Trypanosomacruzi infection. C3H/HeN mice were immunized with Salmonella pCDNA (control) or Salmonella carrying plasmids encoding cruzipain (Cz) and Tc52 (SCz+STc52) or with Cz, Tc52, and Tc24 (SCz+STc24+STc52). Animals were challenged 15 days after the last boost with 1000 trypomastigotes by intraperitoneal route and further evaluated. A, Protection during the acute phase of the infection: (I) parasitemia, (II) weight loss, and (III) survival. All comparisons were referred to the control group. B, Protection during the chronic phase of the infection. Blood was collected on day 100 post-infection, and assays were performed to determine the levels of creatinine kinase (CK, I), lactate dehydrogenase (LDH, II), and aspartate transaminase (AST, III). The bars represent the average ± standard error of the mean. Differences were significant with respect to the control group at *P < .05 and **P < .01. Abbreviation: dpi, days post-infection.
DISCUSSION
After several years of debate about the pathogenesis of Chagas disease, it has been accepted that preventing infection or controlling the acute parasite load would be effective in decreasing tissue damage. This provides new incentive for the development of prophylactic vaccines against T. cruzi infection. Accordingly, several antigens have been tested in animal models as vaccine candidates against T. cruzi [28]. In parallel, efforts to enhance the protective efficacy of subunit vaccines have included testing of adjuvants [29, 30] or antigen-DNA delivered by bacteria or viruses [31], including heterologous prime-boost protocols [32]. In this context, we previously reported the ability of attenuated S. enterica carrying a eukaryotic vector encoding Cz DNA to promote a Th1 protective response against T. cruzi challenge [10].
Here, we determined if the degree of protection conferred by this DNA-based vaccine could be further increased by incorporation of additional antigens. To this end, we assessed the efficacy of a multicomponent approach based on the coadministration of Salmonella carrying eukaryotic vectors coding for Cz, Tc52, and Tc24. The antigen selection was based on the fact that they are immunogenic in natural infection [33–35], important in different metabolic and/or pathogenesis processes [36], present in the parasite membrane or released [37, 38], phylogenetically conserved in diverse T. cruzi strains [39], present in the 3 main developmental stages of the parasite [39], and able to induce protection in prophylactic and/or therapeutic settings [17, 40, 41].
Mice vaccinated by oral route with the multicomponent vaccine (SCz+STc24+STc52) showed no antigenic interference at systemic or mucosal levels, with significant antibody response against all 3 antigens. The antibodies in sera exhibit biological activity, since they were able to both mediate parasite killing in the presence of complement and limit parasite invasion of eukaryotic cells. These results are more significant considering that overall titers were modest, as is expected for a DNA vaccine. This fact strengthens the hypothesis that the quality of antibodies induced by vaccination against T. cruzi is far more important than the absolute titers. In this regard, we previously demonstrated that low antibody titers against the N-terminal domains of Cz were able to dramatically reduce T. cruzi infection of Vero cells [24]. This has clinical correlations, since it was reported that asymptomatic chronic patients have higher levels of lytic antibodies than those exhibiting clinical disease [42].
Importantly, in mice vaccinated with SCz+STc24+STc52, the cellular immune response to every antigen was not impaired by the presence of the other antigens included in the multicomponent vaccine. Furthermore, 2 major cytokines (IFN-γ and IL-10) were increased in mice receiving SCz+STc52+STc24 (Figure 5). Several studies have demonstrated a correlation between production of pro- and antiinflammatory cytokines by CD4+ T cells with symptomatic and asymptomatic clinical forms of chronic Chagas disease, respectively [43, 44]. IFN-γ–producing CD4+ T cells likely assist phagocytes in parasite killing and provide help for development of CD8+ cytotoxic T lymphocyte effector cells. On the other hand, IL-10+ regulatory T cells are predominantly found in clinically asymptomatic chagasic patients [45], indicating that these cells play an important role by preventing collateral immune damage during T. cruzi infection [46].
Immunization with the multicomponent vaccine promoted efficient protection in mice, which retained their body weight after infection. Animals also showed significantly lower levels of parasitemia than controls or mice immunized with monocomponent formulations (Figure 4). Protection was stable and long lasting, even during the chronic stage of infection. SCz+STc24+STc52–immunized mice showed serum levels of the cardiomyopathy-associated enzymes similar to those of noninfected mice (CK, 27.59 ± 9.6; LDH, 1070 ± 124; and AST, 10.8 ± 3.5; Figure 6). Thus, the multicomponent vaccine showed a clear additive effect considering that better scores were systematically obtained for critical protection parameters such as parasitemia, animal weight, and levels of cardiomyopathy-associated enzymes.
Surprisingly, mice vaccinated with STc24 were not able to restrain T. cruzi infection, showing parasitemia levels similar to those in animals in the control group. The ability of Tc24 to confer immune protection is controversial. An earlier study showed that Tc24 was not a good vaccine candidate, since it induced polyclonal activation of IgM-secreting B cells that facilitate parasite immune evasion [47]. Later, it was reported that immunization or treatment with 2 doses of plasmids encoding Tc24 and TSA-1 with alum induced control of the disease, with reduction of parasitemia, mortality, myocarditis, and heart parasite burden in mice and dogs [17, 18, 20, 48].
Therefore, we determined if, under our experimental conditions, immunization with a bicomponent vaccine including SCz+STc52 would confer similar protection as a trivalent vaccine including Tc24 (SCz+STc24+STc52). No differences were observed between both groups in terms of parasitemia and myocardiopathology. Unexpectedly, most of the mice immunized with the trivalent vaccine showed improved survival post T. cruzi infection during the acute phase compared with those receiving the bivalent vaccine. Considering the immunogenicity profiles and protective efficacy of mono-, bi-, and tri-component vaccines, the underlying mechanisms responsible for the superior efficacy of the tri-component vaccine are unclear. However, the observed improved survival of mice during the acute phase, together with previous evidence on the protective role of the Tc24 antigen in prophylactic or therapeutic vaccines [17, 18, 20, 48, 49], suggest that Tc24 should not be excluded from the multicomponent vaccine. Studies should be conducted to identify parameters that correlate better with parasitemia and myocardiopathology in order to elucidate the mechanisms that lead to improved protection by inclusion of Tc24 in the formulation.
These results confirm that T. cruzi vaccine efficacy is not only highly dependent on the selected antigen(s) but also on the vaccination strategy [10]. Vaccines based on a single antigen are not likely to be able to confer an adequate level of protection against T. cruzi infection. This would be an attainable goal by using a multiantigen approach, which targets several metabolic and pathogenic mechanisms. The experiments described here demonstrate that the Salmonella-based trivalent formulation conferred protection superior to that of the mono- or bivalent vaccines. Although complete parasite clearance would probably not have been achieved, mice immunized with the multicomponent vaccine controlled the parasite burden, resulting in limited inflammatory cells in the heart and absence of tissue destruction during the chronic phase. In this regard, prophylactic vaccination should be effective at preventing Chagas disease. Furthermore, a multicomponent vaccine used in mammals may promote effective control of vectorial transmission to humans. Triatomines that feed on vaccinated mammals with low parasitemia will rarely get infected or will have a decline in the number of trypomastigotes eliminated in their feces.
We conclude that immunity improved when additional antigens were added, highlighting the benefits of a multiantigen approach. Thus, vaccines with a single antigen are not likely to be protective, and a multiantigen approach targeting multiple invasion and metabolic mechanisms is likely required to better protect against T. cruzi infection and disease.
Notes
Acknowledgment. We are grateful to Blair Prochnow for critical reading of the manuscript.
Financial support. Support was received from Consejo Nacional de Investigaciones Científicas y Técnicas, University of Buenos Aires and Agencia Nacional de Investigaciones Científicas y Técnicas, Argentina PICT-2006-608 PICT-2010-657) the Fogarty International Center TW007972 International Centre for Genetic Engineering and Biotechnology CRP/ARG09-02.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
S. I. C. and M. N. M. contributed equally to this work.




![Biological activity of antibodies elicited in vaccinated mice. C3H/HeN mice were immunized with Salmonella carrying vectors encoding cruzipain (SCz), Tc24 (STc24), Tc52 (STc52), all 3 antigens (SCz+STc24+STc52), or pCDNA 3.1 (+) as control. Two weeks after the last immunization, sera were obtained and evaluated for their activity to mediate (A) complement-dependent trypomastigote lysis and (B) in vitro inhibition of trypomastigotes infection of Vero cells. Cell invasion inhibition was calculated as 100 – [(absorbance of treated infected cells)/(absorbance of untreated infected cells) × 100]. The results are representative of 3 independent experiments. The star at the top of a bar indicates that values were statistically different with respect to those obtained using sera from mice in the control group at *P < .05, **P < .01, and ***P < .001.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/211/5/10.1093_infdis_jiu480/2/m_jiu48002.jpeg?Expires=1716407800&Signature=o6DXIxVdd5ljymJN6Y~wcdvGPIZ87xvhN7G5UU-BLjy-IUKzempb5iZd-6GgD4uDBKVa-AZwfw9Q-lLgNcPr9y58MPt4vyX0Xr8Vwcli42fiihtonrCmwb6y4dHAeum2HZ-kgDz1PodwF0TxRLtY-n3jiM8JiHyunJVsURWKTel-ZXnsnY8aX7xZV~z5BzEltJGcWc7OCCLyWO5zjHWT7dJKnGTHFKVkq3NSM5PjgqS-hRhf~LuKRCiO92wcpve0Orpbzj28mQ4p60f5k0zwpNxTGO653zzA4qho3q~JIWhQoZVqIYhxgukatcJ~-5hf4jHnlp76uMEyCjRsteB9fA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)