-
PDF
- Split View
-
Views
-
Cite
Cite
Sanjay K. Garg, Elisabetta Volpe, Graziana Palmieri, Maurizio Mattei, Domenico Galati, Angelo Martino, Maria S. Piccioni, Emanuela Valente, Elena Bonanno, Paolo De Vito, Patrizia M. Baldini, Luigi G. Spagnoli, Vittorio Colizzi, Maurizio Fraziano, Sphingosine 1–Phosphate Induces Antimicrobial Activity Both In Vitro and In Vivo, The Journal of Infectious Diseases, Volume 189, Issue 11, 1 June 2004, Pages 2129–2138, https://doi.org/10.1086/386286
Close - Share Icon Share
Abstract
Sphingosine 1-phosphate (S1P), a polar sphingolipid metabolite, is involved in a wide spectrum of biological processes, including Ca++ mobilization, cell growth, differentiation, motility, and cytoskeleton organization. Here, we show a novel role of S1P in the induction of antimicrobial activity in human macrophages that leads to the intracellular killing of nonpathogenic Mycobacterium smegmatis and pathogenic M. tuberculosis. Such activity is mediated by host phospholipase D, which favors the acidification of mycobacteria-containing phagosomes. Moreover, when it was intravenously injected in mycobacteria-infected mice, S1P reduced mycobacterial growth and pulmonary tissue damage. These results identify S1P as a novel regulator of the host antimicrobial effector pathways.
Advances in antituberculosis therapy are urgently required for treatment of both the 8–12 million new cases of tuberculosis (TB), which lead to 2 million deaths each year, and for the 2 billion individuals already infected with Mycobacterium tuberculosis, who are at risk of developing disease [1, 2]. The dramatically increasing incidence of antibiotic-resistant M. tuberculosis strains has compromised both the treatment and prevention of this global disease [3]. Thus, the ability to control the enormous health burden of TB will most likely require a detailed understanding of the mechanisms that promote natural immunity to TB and the development of novel therapeutic approaches.
Sphingosine 1-phosphate (S1P), a product of sphingolipid metabolism that is known to regulate the growth, survival, differentiation, and chemotaxis of various cell types, has been suggested to be a unique signaling molecule because of its having both intracellular and extracellular modes of action [4]. Although its intracellular targets have not yet been unequivocally identified [5], data have been reported that S1P may function as a second messenger that is important in the regulation of Ca++ homeostasis [6] and the suppression of apoptosis [7]. Extracellularly, S1P is prevalently associated with serum high-density lipoprotein [8], and it can provide a signal to the target cells through endothelial differentiation gene (EDG) receptors [9]. After binding with cognate receptors, S1P is responsible for much of the lipid-derived biological activity of serum, such as proliferation, survival, formation of stress fiber and focal adhesion plaque, fibronectin matrix assembly, cell rounding, and cell migration [10]. Moreover, S1P has been reported to trigger multiple signaling path-ways, including the activation of phospholipases, such as phospholipase D (PLD) [11], whose role in anti-microbial activity has been reported elsewhere [12]. Finally, the induction of respiratory burst by S1P in alveolar macrophages has also been shown, which suggests that S1P could also play a role as a physiological activator of alveolar macrophages [13].
On these grounds, the role of S1P as a novel regulator of antimicrobial activity has been hypothesized. The results reported here show that S1P induces antimicrobial activity in human macrophages, which leads to the intracellular killing of both nonpathogenic M. smegmatis and pathogenic M. tuberculosis H37Rv. Such mycobacteriocidal activity is mediated by macrophage PLD, which favors the acidification of mycobacteria-containing phagosomes. Finally, experimental infection in mice has shown that in vivo treatment with S1P induces a significant reduction of bacillary load, both in the lung and in the spleen, and of pulmonary tissue damage.
Materials and Methods
Bacteria. Pathogenic M. tuberculosis H37Rv and nonpathogenic M. smegmatis mc2 strains were grown in Middlebrook 7H9 broth supplemented with albumin dextrose catalase. Mycobacteria were then harvested, suspended in sterile PBS (pH 7.2), aliquoted, and stored at −80°C until use. Before infection, aliquots of both strains were grown on 7H10 plates, to titer the bacteria after thawing.
Preparation of macrophages and differentiation of THP-1 cells. Peripheral blood mononuclear cells were isolated from human buffy-coat blood preparations, and monocytes were separated as described elsewhere [14]. To get monocyte-derived macrophages (MDMs), cells were suspended in complete medium (RPMI 1640 supplemented with 10% fetal bovine serum, 2 mmol l-glutamine/L, and 5 µ/mL gentamicine) and then incubated for 6 days in 24-well plates at a concentration of 106 cells/mL. The human promonocytic THP-1 leukemia cell line, having been induced to differentiate, was also used in in vitro experiments, because of its similarity to alveolar macrophages [15]. In particular, cells were grown in complete medium further supplemented with 1 mmol nonessential amino acids/L and 1 mmol sodium pyruvate/L and incubated for 72 h at 37°C in the presence of 20 ng/mL phorbol 12-myristate 13-acetate. Human MDMs or differentiated THP-1 (dTHP1) cells were then washed and reconstituted in complete medium before use in experiments.
Infection and evaluation of mycobacterial growth after in vitro infection. Human MDMs and dTHP1 cells were infected for 3 h with M. tuberculosis at an MOI of 1 or with M. smegmatis at an MOI of 1 or 50, as indicated in the different colony-forming unit experiments. After the removal of nonphagocytosed bacilli, cells were stimulated with either S1P or d-erythro-sphingosine (a synthetic, chemically manufactured product; Calbiochem) at concentrations of 0.5, 5, and 50 µmol/ L, for the times indicated in the different colony-forming unit experiments. To assess the role of macrophage PLD activity in S1P-induced mycobacterial growth control, M. tuberculosis-infected dTHP1 cells were incubated with 5 µmol S1P/L together with different concentrations of ethanol. Short-chain primary alcohols, such as ethanol, inhibit the generation of PLD-dependent phosphatidic acid (PA) and induce the formation of the metabolically inactive phosphatidylethanol (PetOH) by substituting the nucleophilic acceptor of the phosphatidyl moiety for water. The colony-forming unit assays were performed as described elsewhere [16]. In brief, at the indicated time points after infection and stimulation with S1P, cells were lysed using ice-cold sterile PBS that contained 0.1% saponin (Sigma), serially diluted in PBS that contained 0.01% Tween 80 (Merck), and plated in triplicate on Middlebrook 7H10 agar (Becton Dickinson). M. smegmatis and M. tuberculosis colonies were enumerated after incubation of the plates for 3 and 21 days, respectively, at 37°C in humidified air. No modification, in terms of macrophage viability, was detected in any of the experimental conditions used (data not shown).
Analysis of PLD activity and expression. PLD activity in macrophages was measured by use of the transphosphatidylation assay. In brief, 24-well plate-cultured dTHP1 cells were suspended in complete medium supplemented with 20 mmol HEPES/L (pH 7.4) and labeled with 1 µCi/mL [3H]-myristic acid (Amersham), for 120 min at 37°C, followed by washing with RPMI 1640 to remove unincorporated radioactivity. Thereafter, cells were incubated for 15 min at 37°C in complete medium with 0.5% ethanol, to allow the detection of [3H]PetOH as a specific transphosphatidylation reaction product of PLD. Cells were then infected with M. tuberculosis H37Rv at an MOI of 1, for 3 h at 37°C, washed twice, and stimulated or not with 5 mmol S1P/L. At 15, 90, and 180 min after stimulation, cells were washed twice with 20 mmol HEPES/L (pH 7.4), and total lipids were extracted and separated by use of thin-layer chromatography (TLC) in an ethyl acetate:isooctane:acetic acid:water (130:30 :20:100) solvent system, as described elsewhere [17]. Areas that contained [3H]PetOH were collected from silica-gel plates, compared with standard PetOH, and quantified by use of a liquid scintillation spectrophotometer.
The expression of PLD1 was measured by Western blot analysis, as described elsewhere [14]. In brief, dTHP1 cells, cultured in complete medium, were infected or not with M. tuberculosis H37Rv at an MOI of 1, for 3 h at 37°C, washed twice, and stimulated or not with 5 mmol S1P/L at 37°C. At 24 h after stimulation, cells were suspended in lysis buffer (100 mmol Tris-HCl/L [pH 7.4], 2 mmol EDTA/L, 100 µmol NaCl/L, 1% [vol/vol] Nonidet P40, 0.1% SDS, 2000 IU/mL aprotinin, 1 mmol phenylmethylsulfonyl fluoride/L, 100 µmol leupeptin/L, 50 µmol pepstatin A/L, and 10 mg/mL iodo acetamide). Then, 10 mg of total protein was separated onto SDS-PAGE (8% [wt/ vol] gel) and transferred to a nitrocellulose membrane. Filters were then incubated overnight at 4C with a polyclonal anti-PLD1 antibody (Quality Controlled Biochemicals), and the signal was developed by use of an enhanced chemiluminescence substrate for horseradish peroxidase and then exposed to CLXposure film (Pierce).
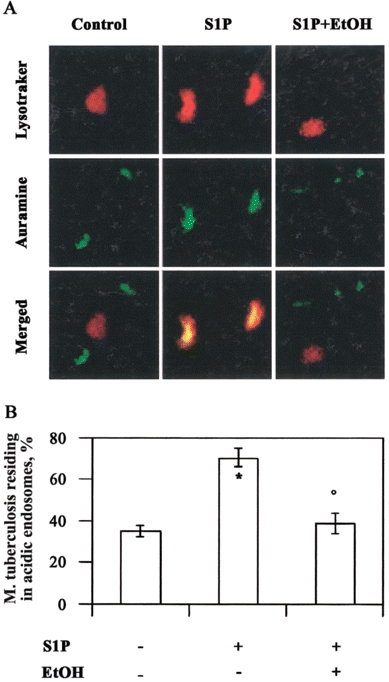
Confocal fluorescent microscopy. The degree of maturation of M. tuberculosis-containing phagosomes was assessed by analyzing the colocalization of bacilli with lysosomes after mycobacteria were stained with auramine and lysosomes were stained with the acidophilic dye Lysotracker Red (Molecular Probes). The dTHP1 monolayer, suspended in complete medium that contained 20 mmol HEPES/L (pH 7.4), was incubated with Lysotracker Red at a 1:10,000 dilution for 2 h at 37°C. Unincorporated dye was removed, and cells were infected for 3 h with M. tuberculosis. After the removal of nonphagocytosed bacilli, Lysotracker Red was added again to each well for 30 min. Cells were washed and incubated for 3 h with 5 mmol S1P/L in the presence or absence of 0.3% ethanol. Thereafter, the monolayer was washed with PBS, .xed by 15 min of incubation with 3.75% paraformaldehyde at room temperature, and permeabilized with ice-cold methanol:acetone (1:1), followed by 3 further washings with PBS. Cells were then seeded on slides pretreated with poly-l-lysine (Sigma). The localization of M. tuberculosis was determined by incubating the infected monolayer with auramine (Becton Dickinson) for 20 min at 25°C, followed by 3 min of incubation in 0.5% acid alcohol and repeated washing with PBS. Finally, cover slips were mounted with Vectashield mounting medium H-1000 (Vector Laboratories), and edges were sealed with nail polish. Confocal fluorescence microscopy was performed on a Zeiss Laser Scan Inverted 510 microscope (Carl Zeiss). An argon-krypton laser (excitation, 488 nm; emission band pass, 505–530 nm) was used for the detection of auramine fluorescence, and a helium-neon laser (excitation, 543 nm; emission limit of pass, 585 nm) was used for the detection of Lysotracker Red. Neither S1P nor ethanol directly affected the fluorescence of auramine or Lysotracker Red (data not shown).
Experimental infection in mice. BALB/c mice (female, 6 weeks old; Charles River Laboratories) were maintained in a biosafety level 3 facility. The mean ±SD temperature (20°C ± 2°C) and humidity ( 55% ± 5% relative humidity) were continuously monitored, and food (RF-18; Mucedola) and water were given ad libitum. Four mice were used in each experimental group throughout the experiments. Mycobacteria were suspended at 5 . 106 cfu/mL in sterile saline, and 106 cfu of either M. smegmatis or M. tuberculosis H37Rv was injected via the retroorbital vein. M. smegmatis-infected mice received 1, 5, or 20 nmol of S1P through the same route at 90 min, and M. tuberculosis-infected mice received the same doses of S1P on days 1 and 7 after infection. M. smegmatis- and M. tuberculosis-infected mice were then killed on days 3 and 21 after infection, respectively. Lungs and spleens were collected and homogenized in sterile saline, and mycobacteria were enumerated by use of the colony-forming unit assay described above. The lower lobe of the left lung was removed from each mouse and used for histological analysis. The Animal Care and Use Committee of University of Rome “Tor Vergata” approved all experiments.
Histological analysis. Lung specimens from all 4 mice in each experimental group were fixed in 10% buffered formalin and embedded in paraffin, and 4-µm sections were stained with hematoxylin-eosin and examined for the formation of granulomas. In particular, to quantify the extension of the granulomatous reaction, the entire surface of the lower lobe of left lung was analyzed. Moreover, areas of both granulomatous and healthy pulmonary parenchyma were evaluated by use of Scion Image Analysis software (Scion). The presence of M. tuberculosis inside lung granulomas was evaluated by auramine staining, according to the manufacturer's indications (Becton Dickinson), and M. tuberculosis were automatically quantified, using Scion Image Analysis software, by analyzing 20 granulomas/ experimental group.
Statistics. Comparison between groups was done using Student's t test, as appropriate for normally distributed data. The Mann-Whitney U test was performed for data that were not normally distributed. P < .05 was considered to be statistically significant.
Results
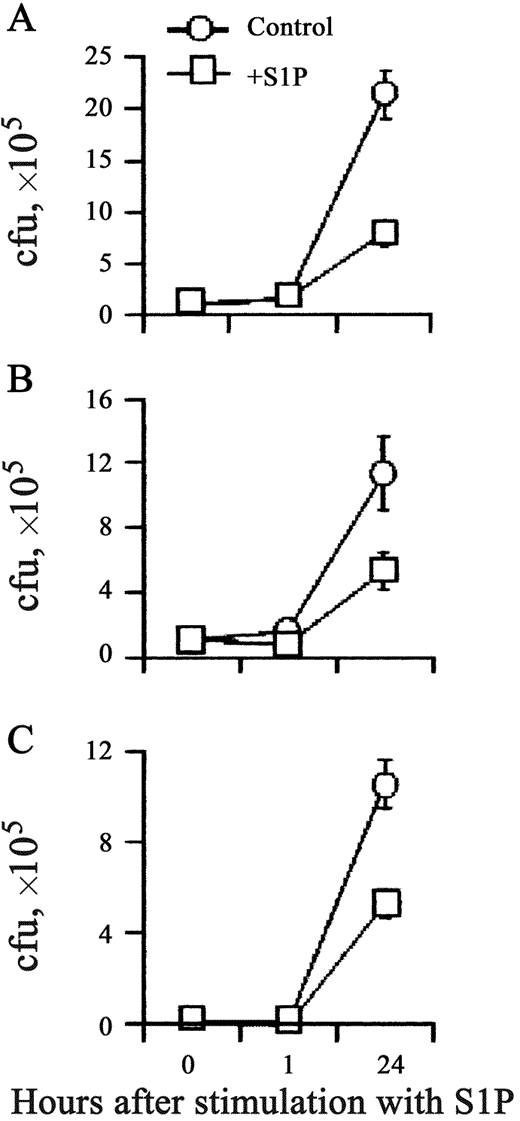
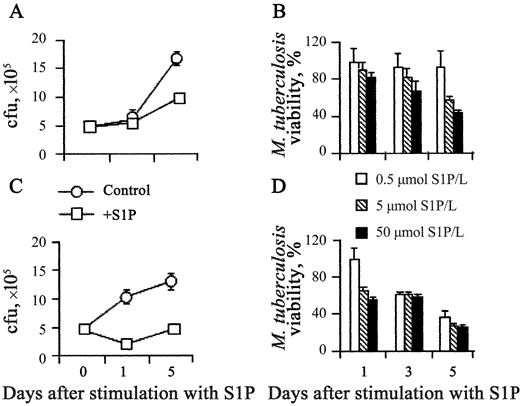
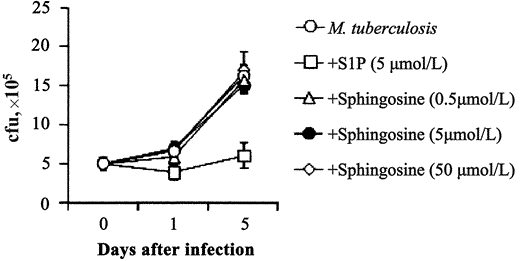
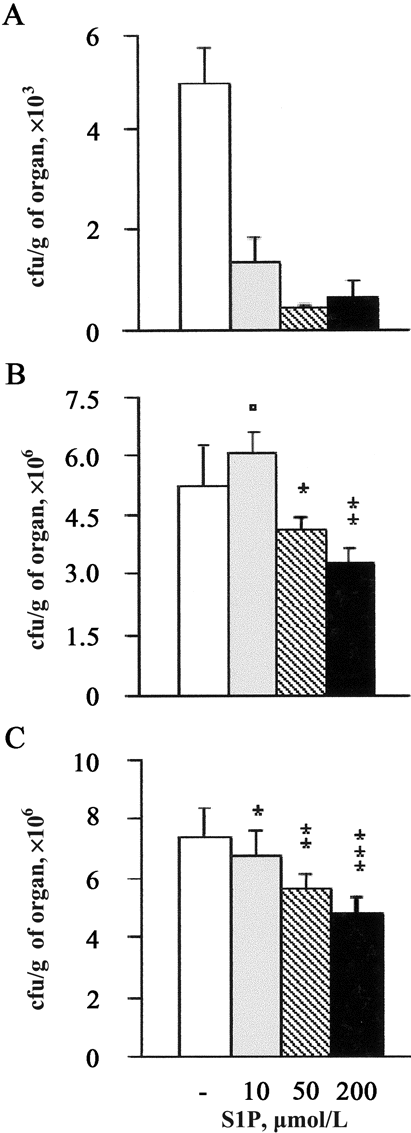
S1P-induced antimycobacterial activity in human macrophages. Adherent macrophages are permissive for the intracellular growth of nonpathogenic mycobacterial strain M. smegmatis [18]. To assess whether S1P is able to increase antimicrobial activity in macrophages, human MDMs and dTHP1 cells were infected with M. smegmatis, and mycobacterial viability was monitored by use of a colony-forming unit assay at 0, 1, and 24 h after stimulation with S1P. Results expressed in figure 1 show that S1P induced the control of intracellular M. smegmatis growth after infection at low MOIs in both MDM (figure 1A) and dTHP1 (1B) cells and at high MOIs in dTHP1 cells (figure 1C). To test whether S1P was able to also induce antimicrobial activity against virulent strain M. tuberculosis H37Rv in macrophages, intracellular mycobacterial growth in MDMs and dTHP1 cells was analyzed on days 1, 3, and 5 after stimulation with S1P. significant inhibition of intracellular mycobacterial growth was observed after stimulation with 5 mmol S1P/L in both MDM (figure 2A) and dTHP1 (figure 2C) cells. The results of the analysis of dose-dependent response showed a progressive increase in antimicrobial activity that indicated a mean 57% reduction in mycobacterial viability when the highest dose of S1P was used and a mean 75% reduction when either 5 or 50 µmol S1P/L was used, as determined on day 5 after infection of MDMs (figure 2B) and dTHP1 cells (figure 2D), respectively. No direct effect of S1P on mycobacterial viability was observed (data not shown). Finally, 3 different doses of sphingosine were tested as a specificity control. Figure 3 shows that only S1P induced antimicrobial activity in MDMs.
Inhibition of the intracellular growth of Mycobacterium smegmatis in human macrophages by sphingosine 1-phosphate (S1P). Human monocyte-derived macrophages (MDMs) were infected at an MOI of 1 (A) and differentiated THP-1 (dTHP1) cells were infected at an MOI of 1 (B) or 50 (C), then cells were stimulated with 5 mmol S1P/L for 1 and 24 h. Numbers of colony-forming units (cfu) are shown as the mean . SD of triplicate values. MDM data are representative of 5 individual experiments (A), and dTHP1 cell data are representative of 3 different experiments (B and C). A, P = NS and P = .04; B, P = .002 and P = .006; C, P = .001 and P = .01. vs. M. smegmatis-infected control macrophages 1 and 24 h after infection, respectively.
Inhibition of the intracellular growth of Mycobacterium tuberculosis in human macrophages, in a dose-dependent fashion, by sphingosine 1-phosphate (S1P). Human monocyte-derived macrophages (MDMs; A and B) and differentiated THP-1 (dTHP1) cells (C and D) were infected at an MOI of 1 and then stimulated with 5 mmol S1P/L for 1 and 5 days (A and C) or infected with 0.5, 5, or 50 mmol S1p/L for 1, 3, or 5 days (B and D). Nos. of colony-forming units (cfu) are shown as the mean . SD of triplicate values. MDM data are representative of 5 individual experiments (A), and dTHP1 cell data are representative of 3 different experiments (C). Data are expressed as the mean . SD of the percentages of M. tuberculosis viability, calculated in 3 separate experiments performed on both different donor-derived MDMs (B) and dTHP1 cells (D). The percentage of M. tuberculosis viability was calculated as follows: (cfu from M. tuberculosis-infected S1P-treated macrophages/cfu from M. tuberculosis-infected control macrophages) × 100. (A) P = NS and P = .04; (C)P = .007 and P = .0002, vs. M. tuberculosis-infected control macrophages 1 and 5 days after infection, respectively.
Inhibition of the intracellular growth of Mycobacterium tuberculosis in human macrophages by sphingosine 1-phosphate (S1P) but not by sphingosine. Differentiated THP-1 (dTHP1) cells were infected with M. tuberculosis at an MOI of 1 and then stimulated or not with 5 mmol S1P/L or with 0.5, 5, or 50 mmol sphingosine/L. At the indicated times, cells were tested for the intracellular growth of M. tuberculosis by use of a colony-forming unit (cfu) assay. Data are expressed as the mean . SD of triplicate values.
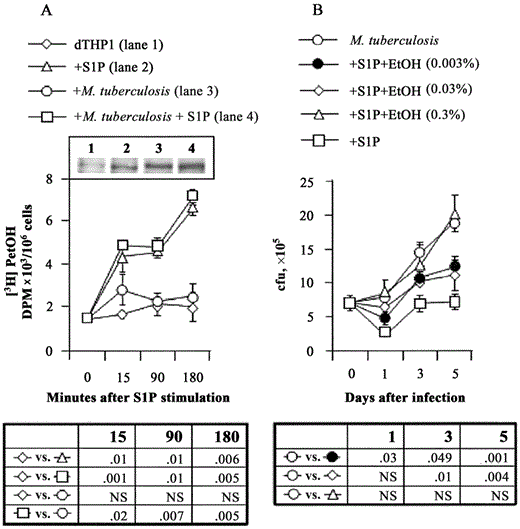
Macrophage PLD-mediated S1P-induced antimicrobial activity. Because human macrophage PLDs have been reported to be involved in the molecular pathway leading to the activation of antimicrobial mechanisms [19] and in adenosine triphosphate (ATP)-induced intracellular mycobacterial killing [20], the activity of PLD after M. tuberculosis infection and stimulation with S1P was tested by use of TLC. Figure 4A shows the formation of [3H]PetOH, as an index of PLD activity, 15, 90, and 180 min after stimulation with S1P. In particular, a progressive and significant increase in PLD activity was observed after with stimulation S1P in both uninfected and M. tuberculosis-infected dTHP1 cells, compared with uninfected and M. tuberculosis-infected dTHP1 control cells, starting 15 min after the studied time points. To test whether a higher level of PLD activity was associated with its higher level of protein expression, the expression of macrophage PLD1 was analyzed in dTHP1 cells 24 h after exposure or not with M. tuberculosis and stimulation or not with S1P. The results reported in the inset of figure 4A show a higher level of protein expression in all experimental conditions, compared with dTHP1 control cells. In particular, stimulation with S1P (lane 2) and M. tuberculosis infection (lane 3) in dTHP1 cells increased the expression of PLD1, compared with control cells (lane 1). M. tuberculosis infection and stimulation with S1P together (lane 4) resulted in the highest immunobinding signal. The involvement of PLD in S1P-induced microbicidal activity was then investigated by inhibiting PLD-mediated PA generation, by use of different concentrations of ethanol. Figure 4B shows that ethanol inhibited the S1P-induced antimycobacterial activity in a dose-dependent manner, reaching it maximum inhibitory effect at a concentration of 0.3%. No direct effect of this amount of ethanol on the growth of M. tuberculosis was observed (data not shown). Similar results were also obtained during the course of S1P-induced control of intracellular M. smegmatis growth (data not shown).
Inhibition of the intracellular growth of Mycobacterium tuberculosis in human macrophages through a phospholipase D (PLD)-mediated mechanism by use of sphingosine 1-phosphate (S1P). A, The formation of phosphatidylethanol (PetOH) was determined by thin-layer chromatography (TLC) and is shown as disintegration per minute values as an index of PLD activity in differentiated THP-1 (dTHP1) cells infected with M. tuberculosis at an MOI of 1 and stimulated with 5 mmol S1P/L for 15, 90, or 180 min. Data are expressed as the mean . SD of triplicate values from 1 representative experiment of 3. Inset A, The expression of PLD1, as analyzed by Western blotting, in uninfected (lane 1), S1P-treated (lane 2), M. tuberculosis-infected (lane 3), and M. tuberculosis-infected S1P-treated (lane 4) dTHP1 cells at 24 h after incubation. B, dTHP1 cells infected with M. tuberculosis at an MOI of 1 and incubated for 1, 3, and 5 days with 5 mmol S1P/L, together with the indicated amounts of ethanol (EtOH). Nos. of colony-forming units (cfu) are expressed as the mean . SD of triplicate values and are representative of 3 separate experiments. Statistical signi.cance is indicated in the table below each panel. NS, not significant.
S1P and acidification of M. tuberculosis-containing phagosomes. Because macrophage PLD is involved in phagolysosome biogenesis [20] and M. tuberculosis is known to reside in nonacidified vacuoles sequestered from late endosomal compartments, the acidification of phagosomes that contain M. tuberculosis in S1P-stimulated cells was investigated by use of confocal fluorescent microscopy (figure 5A). In the absence of S1P, phagocytosed M. tuberculosis bacilli appeared green, which indicated their presence in nonacidic vesicles separate from the red-stained acidic vacuoles. In contrast, treatment with S1P induced a significant increase in the acidification of M. tuberculosis-containing phagosomes, which appeared yellow and indicated the colocalization of green bacilli in acidi.ed phagosomes. The addition of ethanol together with S1P almost completely reverted such processes. Finally, the percentage of M. tuberculosis residing in acidic phagosomes over total intracellular mycobacteria was expressed after serial observations (figure 5B).
Colocalization of Mycobacterium tuberculosis with acidic endosomes after stimulation with sphingosine 1-phosphate (S1P). A, Representative picture from 3 separate experiments showing the increase of M. tuberculosis in acidic vacuoles after treatment with S1P and the reverse effect exerted by ethanol (EtOH). B, Summary of the mean percentage . SE of M. tuberculosis colocalized with acidic phagosomes, determined by counting 1150 bacilli from at least 60 macrophages/sample. Three different experiments were assessed. * P < .0001 and °P = NS, vs. M. tuberculosis-infected control cells.
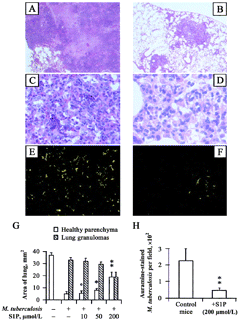
S1P and decreased mycobacterial outgrowth and granulomatous response in infected mice. To test the antimicrobial activity of S1P in vivo, we performed experimental infection of mice with M. smegmatis or M. tuberculosis. A 10-fold reduction in mycobacterial burden was observed in the lungs of mice infected with M. smegmatis (figure 6A) at the intermediate dose of 5 nmol/mouse. Moreover, treatment of S1P reduced the M. tuberculosis burden in a dose-dependent manner both in the lung (figure 6B) and in the spleen (figure 6C), reaching ∼47% mycobacterial growth inhibition after treatment with the use of 20 nmol/mouse. The results of histological analysis showed an extensive granulomatous reaction, with caeseous necrosis in the lungs of M. tuberculosis-infected mice (figure 7A), which was characterized by a mixed neutrophil, macrophage, and lymphocyte infiltrate (figure 7C) and an extensive mycobacterial burden (figure 7E and 7H). A significantly lower granulomatous reaction, with no evidence of necrosis (figure 7B), characterized by a prevalent macrophage infiltrate (figure 7D) and a highly significant reduction in the number of auramine-stained mycobacteria (figure 7F and 7H), was observed after treatment with S1P at the highest dose (20 nmol/ mouse). The quantitative analysis of tissue damage showed a dose-dependent recovery of pulmonary parenchyma that increased up to 3-fold after treatment with the highest dose (20 nmol/mouse), which corresponds to an ∼50% recovery of the total pulmonary area analyzed (figure 7G). Finally, treatment with S1P did not affect the body weight of mice, compared with age-matched, S1P-untreated, M. tuberculosis-uninfected control mice (data not shown).
Reduction of mycobacterial load during the course of in vivo mycobacterial infections by sphingosine 1-phosphate (S1P). Mice were infected with either Mycobacterium smegmatis or with M. tuberculosis H37Rv and treated or not with 1, 5, or 20 nm of S1P. Lungs (A) from M. smegmatis-infected mice and lungs (B) and spleen (C) from M. tuberculosis-infected mice were collected, and nos. of colony-forming units (cfu) were determined in triplicate. Data are expressed as the mean . SD of cfu collected from 4 different mice/group. P values were calculated vs. S1P-untreated mycobacteria-infected mice. A, P < .0001 in all S1P doses tested; B, P = NS, *P = .01, and **P = .001; C, *P = .03, **P = .0002, and ***P = .0001.
Morphological examination of lung tissue in Mycobacterium tuberculosis-infected mice (A, C, and E) and in M. tuberculosis-infected sphingosine 1-phosphate (S1P)-treated mice (B, D, and F). In particular, almost all the lung parenchyma was occupied by granulomas in M. tuberculosis-infected mice (A, magnification 200×), which were characterized by a necrotic core, a polymorphous in.ammatory infiltrate (C, magnification 800×) and disseminated auramine-stained mycobacteria (E, magnification 2000×). Treatment with S1P induced a dose-dependent reduction of lung granulomas (B, magnification 200×) that was characterized by a prevalent macrophage infiltrate without a significant necrotic reaction (D, magnification 800×) and a smaller no. of auramine-stained mycobacteria (F, magnification 2000×). G, Summary of the areas of healthy lung parenchyma (empty column) and of granulomas (dashed column), expressed as mean ± SD, evaluated by analyzing 10 fields covering the entire lobe surface from 4 mice/experimental group. H, Summary of the mean ± SD of the no. of auramine-stained bacilli observed on 20 granulomas/experimental group. °P = NS, *P = .02, and **P < .0001, vs. M. tuberculosis-infected S1P-untreated mice.
Discussion
The dynamic interaction between M. tuberculosis and human MDMs plays a key role in determining the outcome of infection. In this context, the identification of physiological ligands and the elucidation of the molecular mechanisms that lead to the control of mycobacterial intracellular growth represent an important goal for TB research. In fact, no physiological agonists have yet been reported that can unambiguously stimulate the anti-TB activity of macrophages both in vitro and in vivo, although conflicting data regarding several cytokines, immunomodulators, and other inflammatory mediators have been presented. Moreover, the specific signaling pathways that regulate and the effector mechanisms that mediate the killing of intracellular M. tuberculosis have been poorly elucidated [21]. The present study has reported a novel role of S1P as a regulator of antimicrobial activity, exerted both in vitro and in vivo against nonpathogenic M. smegmatis and pathogenic M. tuberculosis infection. Of interest, the results reported here show that dTHP1 cells are more sensitive to S1P signaling, in terms of both dose-dependent responsiveness and a reduction in mycobacterial viability, compared with MDMs. This evidence may be related to the different expression of S1P receptors in either host cell—this possibility is currently under investigation. The experimental use of M. smegmatis as an “absurd” model for TB research has been recently debated [22]. It may survive and multiply inside adherent macrophages [18]. Because M. smegmatis is fast growing and transformable, it is being used as a host to manipulate and express M. tuberculosis and M. avium genes [23] that retain unique mycobacterial determinants. The comparative in vitro and in vivo analysis of the S1P-induced antimicrobial effect against both mycobacteria supports the idea that fast-growing mycobacteria, such as M. smegmatis, may represent a useful experimental model from which to derive preliminary and rapid information regarding the molecular mechanisms involved in macrophage antimycobacterial activity.
Exogenous S1P has been reported to induce PLD activity that leads to the formation of intracellular PA [11], which is involved in the generation of a number of macrophage antimicrobial activities, such as phagocytosis [19], the production of reactive oxygen intermediates (ROIs) [24], the intracellular trafficking of endocytosed immunocomplexes to lysosomes [25], and phagolysosome maturation during the course of ATPinduced mycobacterial killing [20]. On these grounds, the role of PLD in S1P-induced antimicrobial activity was hypothesized. Our results show that S1P-induced antimicrobial activity involves the activation of macrophage PLD, which favors the progressive acidification of mycobacteria-containing phagosomes and M. tuberculosis killing. Support for a key role of PLD in S1P-induced mycobacteriocidal activity in human MDMs consisted of the (1) elevation of [3H]PetOH formation as a metabolically stable index of PLD activity, (2) increased PLD1 expression, and (3) concordant inhibition of mycobacterial killing, using ethanol as a specific inhibitor of PLD-mediated generation of PA. Finally, the role of PLD-mediated phagolysosome maturation in the course of S1P-induced control of mycobacterial growth was analyzed.
In this context, the molecular probe Lysotracker Red is a weak base study phagosome acidification because it is selectively taken up within cells by acidic organelles. In the present study, Lysotracker Red targeted phagosomes that contained M. tuberculosis after stimulation with S1P, which suggests that phagolysosome maturation was progressing. It is still not known whether PA, a known fusogen to the endosome membrane [26], can directly promote endosome membrane fusion or whether the effect is mediated by some other downstream effector mechanism. In fact, PLD may also activate additional phospholipases, such as phospholipase A2 (PLA2), which can favor endosome fusion through the generation of arachidonic acid [27]. To this regard, evidence has been reported that shows that S1P may induce the formation of arachidonic acid through a signaling pathway that involves EDG receptors, PLD, and PLA2 [28].
The success of M. tuberculosis as a pathogen resides in its ability to persist and replicate within the hostile environment of macrophages. A number of pathogenic mechanisms have been reported to contribute to intracellular mycobacterial survival, including the avoidance of ROIs and the generation of reactive nitrogen intermediates, HLA class II down-regulation [21], and the blockage of both Ca++ signaling and phagolysosome maturation via the inhibition of sphingosine kinase [29]. Furthermore, it has been reported that the stimulation of EDG receptors by exogenous S1P induces the formation of intracellular S1P, which leads to the release of Ca++ from internal stores [30]. In this context, our results show that stimulation with S1P, in both M. tuberculosis-infected and -uninfected macrophages, induces the expression of PLD1 and of enzymatic activity, whereas M. tuberculosis alone, despite the increased protein expression, does not induce any significant enzymatic activity. This observation suggests possible mycobacterial interference with the macrophage metabolism that occurs during the course of infection. A blockage of the Ca++ signal by M. tuberculosis and a requirement of Ca++ for PLD activation have been reported [29, 31]. Thus, it is possible to hypothesize that the addition of S1P induces higher PLD activity by increasing PLD1 expression and by restoring required signaling, which leads to the activation of PLD and phagolysosome maturation. Although further investigations are needed to more thoroughly assess the molecular pathways involved in S1P-induced mycobacterial killing, our results show that the increased expression of PLD1 may contribute to the increased S1P-induced PLD activity and support the idea that PLD plays a key role in macrophage-mediated antimycobacterial activity.
S1P has been shown to play a protective role in disease states such as atherosclerosis [32] and certain cancers [33, 34]. Moreover, it has been reported to induce the secretion of (1) interleukin (IL)-6 by airway smooth-muscle cells [35], (2) IL-8 by bronchial epithelial cells [36], and (3) IL-1β and tumor necrosis factor-α by macrophages [37]. However, S1P can also inhibit IL-12 and increase the production of IL-10 in maturing dendritic cells activated with microbial products; therefore, it favors a shift toward a Th2-mediated immune response [38]. Furthermore, S1P and other S1P receptor agonists have recently been described to induce lymphopenia in blood and thoracic duct lymph by the sequestration of lymphocytes in lymph nodes, which suggests their potential role in immunosuppressive therapy [39]. Our results show that M. tuberculosis-infected S1P-treated mice had significant reduction in mycobacterial burden and granulomatous reactions, with prevalent macrophage infiltrates and no evidence of necrotic areas, which suggests that S1P can reduce tissue-damaging T cell-mediated immune responses and simultaneously increase the local innate antimicrobial activity exerted by macrophages. In this context, mouse macrophages have been reported to express EDG1 and EDG5 S1P receptors [37], which suggests the involvement of S1P in the regulation of pulmonary in.ammatory responses. Finally, S1P has also been reported to be an important angiogenic factor in serum [40] and an enhancer of endothelial barrier integrity [41]. These may have important implications, because the tissue-damage immune response is also associated with the loss of endothelial barrier integrity, which may, in turn, be responsible for the hematogenous dissemination of mycobacteria.
One of the puzzling aspects of TB is that, although one-third of the world's population is infected, <10% of infected individuals develop active disease during the first few years after exposure [42], which suggests that components of the natural immune response may play an important role in protection. Moreover, there is strong evidence that primary malnutrition raises the incidence and exacerbates clinical manifestations of TB [43]. Within this context, the requirement of S1P in the control of primary M. tuberculosis infection can be hypothesized. Studies with animal models have shown that feeding sphingolipid to mice inhibits colon carcinogenesis [44] and reduces the risk of atherosclerosis by decreasing plasma cholesterol levels [45], which suggests that sphingolipids may represent a “functional” constituent of food [46]. On these grounds, it is suggestive to hypothesize a nutritional value of either S1P or its precursors, which can contribute to the antimicrobial immune surveillance of the host and, hence, to prevent active disease among the developing-world population that harbors latent M. tuberculosis.
References
Figures and Tables
Financial support: Italian Ministry of University; IV Research Program on AIDS (grant 50D.05) of the Italian Institute of Health; UNESCO-ROSTE (study fellowships to G.S.K., V.E., and G.D.).