-
PDF
- Split View
-
Views
-
Cite
Cite
Carolyn A. Lacey, Lauren L. Keleher, William J. Mitchell, Charles R. Brown, Jerod A. Skyberg, CXCR2 Mediates Brucella-Induced Arthritis in Interferon γ–Deficient Mice, The Journal of Infectious Diseases, Volume 214, Issue 1, 1 July 2016, Pages 151–160, https://doi.org/10.1093/infdis/jiw087
Close - Share Icon Share
Abstract
Background. Brucella species are facultative intracellular gram-negative bacteria that cause brucellosis, a common global zoonosis. Infection of the joints is the most common focal complication of brucellosis in humans. The purpose of this study was to identify mediators of focal inflammation during brucellosis.
Methods. Wild-type (WT) mice are naturally resistant to Brucella infection; therefore, we infected anti-interferon γ (IFN-γ)–treated, or IFN-γ−/− mice with Brucella to induce osteoarticular and musculoskeletal inflammation, as we previously described. Mice were infected intraperitoneally with Brucella melitensis, and the clinical course of disease, histopathologic changes, and cytokine levels were compared among groups.
Results. Rag1−/− mice (B- and T-cell deficient) and µMT−/− mice (B-cell deficient) developed paw inflammation at a similar rate and severity as WT mice following infection with B. melitensis and treatment with anti–IFN-γ. Joints from B. melitensis–infected IFN-γ−/− mice had markedly increased levels of CCR2 and CXCR2 ligands. While anti-IFN-γ–treated CCR2−/− and WT mice behaved similarly, anti-IFN-γ–treated CXCR2−/− or IFN-γ−/−/CXCR2−/− mice had strikingly reduced focal swelling relative to anti-IFN-γ-treated WT or IFN-γ−/− mice, respectively. Additionally, neutrophil recruitment was dependent on CXCR2.
Conclusions. Adaptive immune cells and CCR2 are dispensable, while CXCR2 is necessary for Brucella-induced focal neutrophil recruitment and inflammation.
Brucellosis, caused by Brucella species, is one of 8 neglected zoonoses according to the World Health Organization [1]. Also known as undulant fever, brucellosis remains a major global health, agricultural, and economic problem. With >500 000 new cases annually, brucellosis is one of the most common zoonotic diseases worldwide [2]. While generally not life threatening, brucellosis can cause disease with relapses and lifelong complications, including arthritis, endocarditis, and possible neurological disorders [3, 4].
Osteoarticular complications, found in 40%–80% of infected patients, are the most common focal symptoms of acute and chronic brucellosis [5, 6]. Arthritis can manifest as peripheral arthritis, sacroiliitis, and spondylitis [5–8]. Peripheral arthritis generally affects weight-bearing joints such as the hips, knees, and ankles and develops clinically with soft-tissue swelling and periarticular osteoporosis [5, 9]. Brucellar arthritis generally responds to antibiotics; however, complete resolution of inflammation is often prolonged [6, 10] and can result in permanent osteoarticular damage [8].
Several Brucella species are known to cause osteoarticular complications in humans, including Brucella melitensis, Brucella abortus, and Brucella suis [6, 11, 12]. Investigation of articular brucellosis has been hindered by the lack of relevant models, as wild-type (WT) mice infected with conventional doses of Brucella do not develop disease in the same way as humans or ruminants [13, 14]. Others have shown that high-dose intraperitoneal infection of mice with B. abortus (107 colony-forming units [CFUs]) can result in osteoarticular complications by 26 weeks after infection [15]. In our own work, we have shown that genetic or antibody-mediated ablation of interferon γ (IFN-γ) in mice results in joint colonization and development of arthritis 2–4 weeks after intranasal or intraperitoneal infection with approximately 2 × 104 CFUs B. melitensis or B. abortus [13]. Although a human might have an intact IFN-γ gene, a single-nucleotide polymorphism (+874A/A) that potentially subverts optimal nuclear factor κB binding to the IFN-γ gene and correlates with diminished IFN-γ expression was found to be a brucellosis risk factor [16–18]. In addition, another IFN-γ polymorphism (5644A) associated with low IFN-γ production was found to be overrepresented in patients with focal complications of brucellosis [19]. Patients with chronic brucellosis also have an impaired IFN-γ response to Brucella antigen relative to patients at the onset of disease [20]. Thus, suboptimal IFN-γ responses may predispose both humans and mice to focal brucellosis.
It has been reported that neutrophils are the most abundant cells in the synovial fluid of mice with experimental rheumatoid arthritis [21, 22]. Examination of synovial fluid or tissue from patients with brucellosis reveals a mixture of polymorphonuclear (PMN) and mononuclear leukocyte infiltrates [6, 23–25]. Neutrophil activation and recruitment to the joints is stimulated by proinflammatory cytokine/chemokine secretion into the synovium, consequentially leading to neutrophil release of granule contents, reactive oxygen species, and other proinflammatory products that may drive inflammation [21].
Little information exists on the composition of cytokines/chemokines in the synovial fluid of patients with brucellosis. Previously, our group found that IL-1R−/− mice have reduced musculoskeletal inflammation when infected with Brucella and depleted of IFN-γ [13]. In addition, elevated levels of interleukin 1β (IL-1β), CCL2, and CXCR2 ligands have been found in the synovial bursa of a patient with brucellosis who had osteoarticular complications [26]. To characterize the induction of Brucella-induced focal inflammation, we investigated the role of adaptive immunity and chemokine production. Here, we show that adaptive immune cells are dispensable but that CXCR2 is critical for Brucella-induced arthritis.
METHODS
Bacterial Strains and Growth Conditions
All experiments with live Brucella were performed in biosafety level 3 (BSL-3) facilities. B. melitensis 16 M, obtained from Montana State University (Bozeman, Montana), was grown on Brucella agar at 37°C (Becton Dickinson). Colonies were picked from Brucella agar plates, and B. melitensis was cultured in Brucella broth overnight at 37°C with shaking. The overnight B. melitensis concentration was estimated by measuring the OD at 600 nm, and the inoculum was diluted to the appropriate concentration in sterile phosphate-buffered saline (sPBS). Actual viable titer was confirmed by serial dilution of the B. melitensis inoculum onto Brucella agar plates.
Mice
Rag1−/−, μmT−/−, CCR2−/− (all on C57BL/6 background), CXCR2−/− (BALB/c background), and breeding pairs of IFN-γ−/− and heterozygous CXCR2+/− mice (both on C57BL/6 background) were obtained from Jackson Laboratory (Bar Harbor, Maine). Heterozygous CXCR2+/− mice were intercrossed with IFN-γ−/− mice to obtain IFN-γ−/−/CXCR2−/− mice. Mice were infected intraperitoneally with 200 µL of approximately 1 × 105 CFUs of B. melitensis 16 M in sPBS for all experiments. In some experiments, mice were treated with anti-IFN-γ antibody (clone XMG1.2, BioXCell) via intraperitoneal administration of 0.25 mg 1 day before infection and 3 times per week thereafter. During infections, all mice were maintained in individually ventilated cages under high-efficiency particulate air–filtered barrier conditions of 12 hours of light and 12 hours of darkness in animal BSL-3 facilities and were provided with sterile food and water. Experiments were conducted with 7–10-week-old age- and sex-matched mice. All studies were conducted in accordance with University of Missouri Animal Care and Use Committee guidelines
Bacterial Burdens
At various times after infection, mice were euthanized, and spleens and mouse paws (following skin removal) were harvested. Spleens and paws were homogenized mechanically in sPBS. A series of 10-fold serial dilutions were performed in triplicate in sPBS and plated onto Brucella agar. Plates were incubated 3–6 days at 37°C, colonies were enumerated, and the number of CFUs/tissue was calculated.
Assessment of Pathology
Paw swelling was monitored every 2–5 days by collective measurements of the tibiotarsal and radiocarpal joints with a metric caliper. Joint measurements were made before infection, and the difference was used to calculate mean joint swelling. Clinical scores were determined using the following scale per paw: 0, no swelling or redness; 1, mild redness or swelling of single digits; 2, swelling of ankle or wrist with erythema; and 3, severe swelling and erythema of multiple joints [13]. For histological analysis, mouse tails were fixed in 10% buffered zinc-formalin, decalcified in 15% formic acid, rinsed in tap water, and dehydrated with an alcohol gradient and clearing agent. Tissue specimens were embedded in paraffin. Tissue sections (5 µm) were mounted on Plus Charged glass slides, and serial sections were stained with hematoxylin and eosin. Enzyme immunohistochemical (IHC) staining was done on deparaffinized sections, using appropriate buffer and rinsing between each immunostaining step. Samples were rehydrated in xylene, absolute alcohol, and 95% alcohol and rinsed with distilled water. Endogenous peroxidase was blocked with 3% H2O2 and 5% bovine serum albumin, and slides were incubated with 4.7 µg/mL of rat anti-Ly-6G antibody (clone 1A8, BioXCell) for 60 minutes. Slides were then incubated with secondary biotinylated donkey–anti-rat antibody, followed with incubation in streptavidin–horseradish peroxidase (Dako). Slides were developed with diaminobenzidine (DAB+, Dako), lightly counterstained with hematoxylin 1, and coverslipped with aqueous mounting medium. Three sections of tail at different tissue levels (bone and associated structures) were evaluated and scored for each mouse by a histopathologist. The following scale was used for total inflammation (based on results of hematoxylin-eosin staining): 0, none (no inflammation); 1, minimal with inflammation involving <5% of a tissue specimen; 2, moderate with focally extensive areas of inflammation (5%–25% of a tissue specimen and involving ≥1 tissue level); 3, moderate to severe with focally extensive inflammation areas (>25%–50% of a tissue specimen and involving multiple tissue levels); and 4, severe with large confluent areas of inflammation (>50% of a tissue specimen and involving multiple tissue levels). For neutrophil infiltration (based on IHC findings), the following scoring system was used (labeling of neutrophils in normal bone marrow was excluded from evaluation): 0, none (no labeled cells); 1, minimal numbers of labeled cells; 2, moderate numbers of labeled cells; and 3, large numbers of labeled cells and skeletal muscle along with periarticular inflammation.
Paw Homogenization for Cytokine Measurement
Following skin removal, paws from each mouse were detached, combined, and then homogenized in toto in 3–4 mL of sPBS treated with Halt Protease Inhibitor Cocktail (Thermo Scientific) [13]. Homogenized tissues were then centrifuged at 2000g for 5 minutes, and supernatants were filter sterilized (0.2 µm) and stored at −70°C until analysis using a Luminex MagPix instrument with Milliplex magnetic reagents according to manufacturer's instructions (Millipore). Luminex data was analyzed with Milliplex Analyst Software (Millipore) and normalized to total protein levels determined by a bicinchoninic acid protein assay (Thermo Scientific).
Flow Cytometry Analysis of Joints
Rear legs underwent degloving, hip dislocation, and muscle trimming; were put into a sterile 4-mL PBS solution containing 125 U/mL collagenase (Sigma), 2 µL/mL of DNase (l U/mL; Thermo Scientific), and 4% fetal bovine serum (FBS; Sigma); and were incubated for 1 hour at 37°C with shaking. Joint slurry was put into sterile dishes, stripped of surrounding tissue, strained through an 80-µM mesh, and washed with complete medium (Roswell Park Memorial Institute [RPMI] 1640 medium, 0.1 mM HEPES, 1 mM sodium pyruvate, 1 mM nonessential amino acids, and 10% FBS). Cells were resuspended in RPMI 1640 medium with 0.1 mM HEPES, and red blood cells were lysed with lysis buffer (final concentration, 0.15 M NH4Cl, 10 mM KHCO3, and 0.1 mM ethylenediaminetetraacetic acid) [27]. Cells were then washed and stained in fluorescence-activated cell-sorting (FACS) buffer (PBS and 2% FBS). Immunofluorescence staining was performed for 30 minutes at 4°C, using the following fluorochrome-labeled monoclonal antibodies from eBioscience: F4/80 (BM8), Ly-6G (1A8), CD11b (M1/70), CD45.2 (104), CD4 (GK1.5), CD8a (2.43), and B220 (RA3-6B2). Stained cells were then washed with FACS buffer and fixed overnight in 4% paraformaldehyde at 4°C. Fluorescence was acquired on a CyAn ADP Analyzer (Beckman Coulter), and FlowJo (Tree Star) software was used for analysis.
Statistical Analysis
Focal inflammation incidence was analyzed by log-rank analysis on incidence curves. Statistical analysis of the difference between 2 mean values was conducted using the Student t test with significance set at P ≤ .05, while comparisons of ≥3 mean values was done using a 1-way analysis of variance, followed by the Tukey test. The statistical significance of differences in histology scores was determined by a Mann–Whitney U test.
RESULTS
Inflamed B. melitensis–Infected Joints Have Increased Levels of Neutrophils
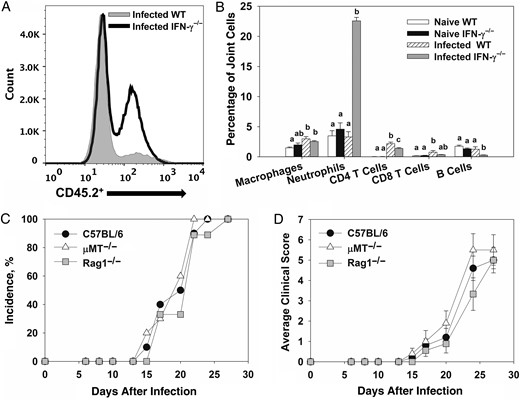
To characterize infiltrating cells, flow cytometry was performed on joints from B. melitensis–naive and B. melitensis–infected mice. A marked increase in levels of hematopoietic cells (CD45.2+), particularly neutrophils (CD45.2+/Ly-6Ghi), was observed in Brucella-infected IFN-γ−/− mice (Figure1A and 1B), relative to infected WT mice. To a lesser extent, there was a slight but statistically significant increase in the number of CD4+ T cells in Brucella-infected WT and IFN-γ−/− mice relative to the number in naive controls (Figure 1B). A significant increase in the proportion of CD45.2+/CD11b+/Ly6-G−/F4/80− cells was also seen in infected IFN-γ−/− mice, relative to infected WT animals, which may indicate an influx of monocytes (data not shown).
T and B cells are not required for Brucella-induced focal inflammation. A–B, Flow cytometry was performed on joints from naive C57BL/6 and interferon γ (IFN-γ)−/− mice or from C57BL/6 and IFN-γ−/− mice 28 days after intraperitoneal infection with approximately 1 × 105 colony-forming units (CFUs) of Brucella melitensis 16 M (4–5 mice/group). Within a cell type, mean values with the same letter are not significantly different from each other (P < .05). Error bars depict standard errors of the mean (SEMs). C–D, Wild-type (WT), μMT−/−, and Rag1−/− mice (all on a C57BL/6 background) were infected intraperitoneally with approximately 1 × 105 CFUs of Brucella melitensis 16 M and treated with anti-IFN-γ (5–10 mice/group). Incidence of paw swelling (C) and average clinical scores (D) were recorded over time. Error bars depict SEMs.
T and B Cells Are Not Required for Brucella-Induced Focal Inflammation
To investigate a role for adaptive immune cells, WT, μMT−/− (B-cell deficient), and Rag1−/− mice (B- and T-cell deficient) were infected intraperitoneally with approximately 1 × 105 CFUs of B. melitensis and treated with anti-IFN-γ antibody as described in “Methods” section. To assess inflammation, mice were monitored for swelling and redness at the tibiotarsal junction. Anti-IFN-γ–treated WT, μMT−/−, and Rag1−/− mice displayed a similar incidence and degree of paw swelling and similar clinical scores (Figure 1C and 1D). Brucella joint tissue loads and neutrophil infiltration, as measured by flow cytometry, were also similar between WT and μMT−/− mice at day 28 after infection (data not shown). Tissue bacterial burdens and joint neutrophil infiltration were not measured in Rag1−/− mice because 80% of animals died from infection by day 28 (data not shown).
CCR2 and CXCR2 Ligands Are Upregulated in Inflamed, B. melitensis–Infected Joints
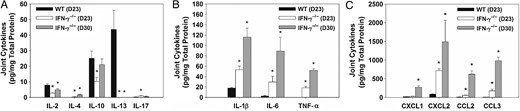
To determine expression of cytokines/chemokines during infection, paw homogenates from B. melitensis–infected WT and IFN-γ−/− mice were assessed for T-cell–associated cytokines, inflammatory cytokines, and chemokines by the Luminex assay 23 and 30 days after infection (Figure 2A–C). In infected WT mice, interleukin 13 and interleukin 2 were the only cytokines with enhanced induction relative to findings in infected IFN-γ−/− animals (Figure 2A). Marked increases in induction of the inflammatory cytokines IL-1β, interleukin 6 (IL-6), and tumor necrosis factor α (TNF-α) were seen in infected IFN-γ−/− mice 23 and 30 days after infection (Figure 2B). Additionally, the chemokines CXCL2, CCL2, and CCL3 were also significantly upregulated on days 23 and 30 in IFN-γ−/− mice as compared to WT mice, while CXCL1 was upregulated at day 30 after infection (Figure 2C).
CCR2 and CXCR2 ligands are upregulated in inflamed, Brucella melitensis–infected joints. Joint homogenates (5 mice/group) from wild-type (WT) and interferon γ (IFN-γ)−/− mice infected intraperitoneally with approximately 1 × 105 colony-forming units of B. melitensis 16 M were assayed for T-cell–associated cytokines (A) inflammatory cytokines (B), and chemokines (C) by the Luminex assay 23 and 30 days after infection and normalized using a bicinchoninic acid protein assay. *P < .05 as compared to WT mice. Error bars depicted standard errors of the mean. Abbreviations: IL-1β, interleukin 1β; IL-2, interleukin 2; IL-4, interleukin 4; IL-6, interleukin 6; IL-10, interleukin 10; IL-13, interleukin 13; IL-17, interleukin 17; TNF-α, tumor necrosis factor α.
CCR2 Is Not a Critical Mediator of Brucella-Induced Focal Inflammation
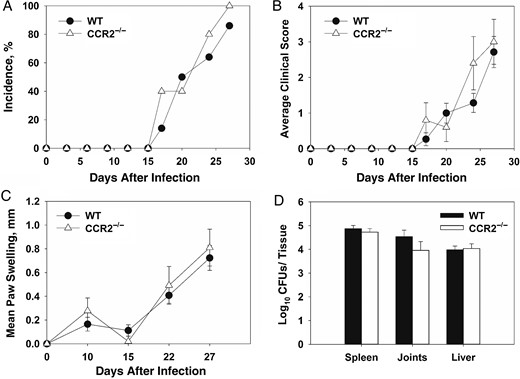
CCR2 is the primary receptor for CCL2 signaling and recruitment of inflammatory monocytes [28]. CCL2 expression was elevated in joint homogenates from infected IFN-γ−/− mice; therefore, we examined the role of CCR2 in Brucella-induced inflammation. WT and CCR2−/− mice (C57BL/6 background) were treated with anti-IFN-γ, infected intraperitoneally with B. melitensis, and monitored over time. Incidence, clinical scores, and levels of swelling were similar in WT and CCR2−/− mice. (Figure 3A–C). Additionally, tissue bacterial loads were comparable in tissues from CCR2−/− and WT mice (Figure 3D).
CCR2 is not a critical mediator of Brucella-induced focal inflammation. Wild-type (WT) and CCR2−/− mice were infected intraperitoneally with approximately 1 × 105 colony-forming units (CFUs) of Brucella melitensis 16 M and treated with anti-interferon γ (IFN-γ; 5–10 animals/group). Incidence of paw swelling (A), clinical scores (B), and mean swelling (C) were recorded over time. On day 28 after infection mice were euthanized, and Brucella CFUs were enumerated (D). Error bars depict standard errors of the mean.
CXCR2−/− Mice Display Reduced Clinical Signs of Brucella-Induced Inflammation
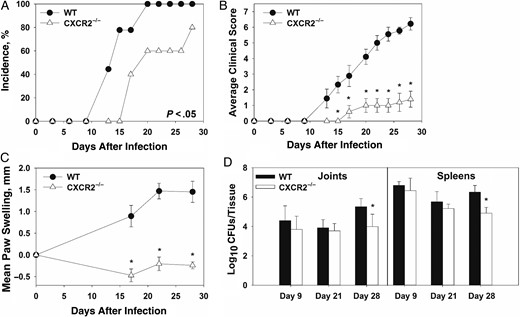
CXCL1 and CXCL2 were upregulated in Brucella-infected joints and are both CXCR2 ligands that can recruit neutrophils to the site of infection [29]. To elucidate the role of CXCR2 in Brucella-induced inflammation, WT BALB/c and CXCR2−/− mice (BALB/c background) were treated with anti-IFN-γ and infected intraperitoneally with approximately 1 × 105 CFUs of B. melitensis. CXCR2−/− mice displayed delayed incidence, reduced clinical scores, and reducing swelling as compared to WT mice (Figure 4A–C). CXCR2−/− mice lost more weight than WT animals (data not shown), which may explain some of the decreased paw thickness in these mice (Figure 4C). Bacterial loads in the spleen and joints of WT and CXCR2−/− mice were similar 9 and 21 days after infection. However, at day 28, CXCR2−/− mice had significantly lower CFUs as compared to WT mice in both the joints and spleen (Figure 4D). We also found that joints from CXCR2−/− mice had reduced levels of CCL2, IL-6, TNF-α, and IL-1β as compared to WT mice 28 days after infection (data not shown).
Reduced focal inflammation in Brucella-infected, anti-interferon γ (IFN-γ)–treated CXCR2−/− mice. Wild-type (WT) and CXCR2−/− mice (both on a BALB/c background) were infected intraperitoneally with approximately 1 × 105 colony-forming units (CFUs) of Brucella melitensis 16 M and treated with anti-IFN-γ. Incidence of paw swelling (A), clinical scores (B), and mean swelling (C) were recorded over time. On days 9, 21, and 28 after infection, Brucella colonization was determined (D). *P < .05 as compared to WT mice. Error bars depict standard errors of the mean (SEMs). Data in panels A–C are from 5–10 mice/group and are representative of 3 independent experiments. CFU data in panel D were obtained from 3–9 mice/group. Error bars depict SEMs.
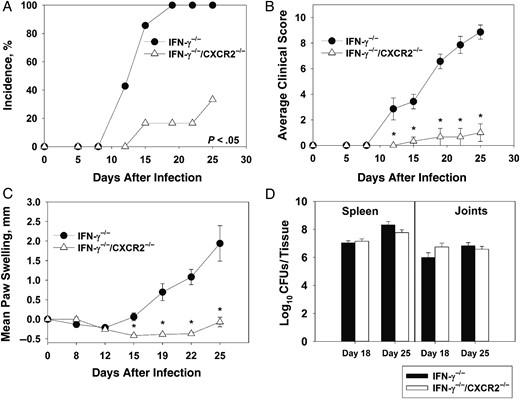
To confirm our results obtained by anti-IFN-γ treatment, we generated IFN-γ−/−/CXCR2−/− mice. Following infection with B. melitensis, IFN-γ−/−/CXCR2−/− mice had delayed arthritis onset, with only 16.6% developing arthritis by day 19 as compared to 100% of IFN-γ−/− mice (Figure 5A). Additionally, IFN-γ−/−/CXCR2−/− mice had reduced clinical scores, with an average score of 1, compared with 8.87 for IFN-γ−/− mice (Figure 5B). Swelling of the paws was also strikingly reduced in IFN-γ−/−/CXCR2−/− mice (Figure 5C). IFN-γ−/−/CXCR2−/− mice lost more weight than IFN-γ−/− mice; however, no significant change in bacterial loads in the spleen or joints between groups was noticed at days 18 and 25 after infection (Figure 5D).
CXCR2 mediates Brucella-induced inflammation in interferon γ (IFN-γ)−/− mice. IFN-γ−/− and IFN-γ−/−/CXCR2−/− mice (on a C57BL/6 background; 4–7 mice/group) were infected intraperitoneally with approximately 1 × 105 colony-forming units (CFUs) of B. melitensis. The incidence of paw swelling (A), clinical scores (B), and mean swelling (C) were recorded over time. Colonization of the joints and spleen was determined on day 18 and 25 after infection (D). *P < .05 as compared to IFN-γ−/− mice. Data in panels A–C are representative of 3 independent experiments. Error bars depict standard errors of the mean.
CXCR2 Mediates Neutrophil Infiltration in Musculoskeletal Tissue
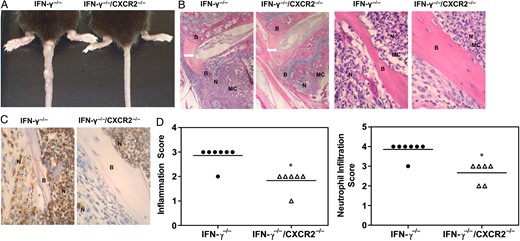
Gross swelling of mouse paws and tails was markedly reduced in IFN-γ−/−/CXCR2−/− mice relative to IFN-γ−/− mice infected with B. melitensis (Figure 6A). Inflammation of mouse paws develops asymmetrically in Brucella-infected IFN-γ−/− mice, while tails typically develop multiple inflammatory foci (Figure 6A). Therefore, to assess inflammation in the same tissue from all animals, histological analysis was performed on mouse tails, and scores were assigned as described in “Methods” section. Hematoxylin-eosin staining of mouse tail sections from B. melitensis–infected IFN-γ−/− mice revealed severe areas of inflammation that involved bone, medullary cavity of bone, tendons, skeletal muscle, and periarticular inflammation (Figure 6B). Osteomyelitis was observed in the medullary cavity of B. melitensis–infected IFN-γ−/− mice, which generally occurred near the growth plate and, in some instances, may have led to erosion of the bone (Figure 6B). Osteomyelitis was also observed in IFN-γ−/−/CXCR2−/− mice infected with B. melitensis but was much milder than that observed in IFN-γ−/− mice. Inflammation was sometimes localized to the medullary cavity in IFN-γ−/−/CXCR2−/− mice, in contrast to IFN-γ−/− mice, which displayed extensive inflammation in bone, tendon, and skeletal muscle (Figure 6B and 6D). Immunochemical analysis was also performed using the neutrophil-specific Ly-6G antibody (clone 1A8). Neutrophil infiltration was seen in both groups, but IFN-γ−/−/CXCR2−/− mice had significantly reduced neutrophil recruitment in inflamed tissues, relative to IFN-γ−/− mice (Figure 6C and 6D). Flow cytometry also revealed an approximately 40% reduction of neutrophil infiltration in joints from IFN-γ−/−/CXCR2−/− mice, relative to IFN-γ−/− mice (data not shown).
CXCR2 mediates musculoskeletal neutrophil recruitment in Brucella-infected mice. Interferon γ (IFN-γ)−/− and IFN-γ−/−/CXCR2−/− mice were infected intraperitoneally with approximately 1 × 105 colony-forming units of Brucella melitensis 16 M and euthanized 25 days after infection. A, Representative tail images comparing IFN-γ−/− and IFN-γ−/− /CXCR2−/− mice. B, Representative images of hematoxylin-eosin–stained, B. melitensis–infected IFN-γ−/− and IFN-γ−/− /CXCR2−/− tail vertebral sections, using a 4× (left) and 40× (right) objective lens. C, Representative images of sections stained for immunohistochemical analysis with anti-Ly6G, (clone 1A8) indicating neutrophil infiltration, using a 40× objective. D, Histopathological scoring of hematoxylin-eosin staining (for determination of total inflammation) and immunohistochemical analysis (for determination of neutrophil infiltration) of tail vertebra sections (6–7/group). *P < .05 as compared to sections from IFN-γ−/− mice. Abbreviations: Arrow, intervertebral disk; B, bone; MC, medullary cavity; N, neutrophil.
Reduced TNF-α and CCL3 Levels in IFN-γ−/−/CXCR2−/− Mice
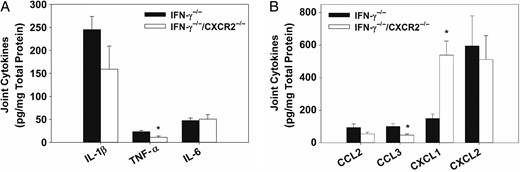
Cytokines from Brucella-infected joints of IFN-γ−/− and IFN-γ−/−/CXCR2−/− mice were evaluated by the Luminex assay to determine signaling pathways that might be regulated by CXCR2. At day 25 after infection, levels of both TNF-α and CCL3 were significantly reduced in joints from IFN-γ−/−/CXCR2−/− mice as compared to those from IFN-γ−/− mice (Figure 7A and 7B). IL-1β levels were also lower, but not significantly so, in IFN-γ−/−/CXCR2−/− mice. Additionally, the antiinflammatory cytokine interleukin 10 was slightly upregulated in IFN-γ−/−/CXCR2−/− mice on day 18 after infection, relative to IFN-γ−/ mice (data not shown).
Reduced levels of tumor necrosis factor α (TNF-α) and CCL3 in interferon γ (IFN-γ)−/− mice lacking CXCR2. IFN-γ−/− and IFN-γ−/−/CXCR2−/− mice were infected intraperitoneally with approximately 1 × 105 colony-forming units of Brucella melitensis. Joint homogenates (6–7/group) were assayed for inflammatory cytokines (A) and chemokines (B) 25 days after infection by the Luminex assay and normalized using a bicinchoninic acid protein assay. *P < .05 as compared to IFN-γ−/− mice. Error bars depict standard errors of the mean. Abbreviations: IL-1β, interleukin 1β; IL-6, interleukin 6; TNF-α, tumor necrosis factor α.
DISCUSSION
Brucellosis is one of the most common zoonotic diseases worldwide [3], and osteoarticular complications are the most frequent localized manifestations [5, 6]. Here, we investigated mechanisms of inflammation during Brucella infection, using our previously published mouse model [13]. We found that T- and B-cell–deficient mice developed focal inflammation similarly to WT animals. We also did not observe robust recruitment of T and B cells to infected joints. In contrast to rheumatoid arthritis, where autoreactive T cells and B cells are the primary cause of pathogenesis [30, 31], T-cell and antibody responses do not appear to be required for Brucella-induced inflammation, similar to what has been reported in other murine models of infectious arthritis, such as Lyme disease [32]. While T and B cells were not required for the development of arthritis in our model, others have shown that Rag1−/− mice are more susceptible to B. melitensis infection [33]. B-cell–deficient mice are more resistant to Brucella infection [34]; however, several other groups have shown that T cells mediate clearance of Brucella and are a major producer of IFN-γ [35, 36]. Thus, in future studies we will determine whether transfer of WT T cells to IFN-γ−/− mice can mediate resolution of Brucella-induced arthritis.
While adaptive immune cells are not required for Brucella-induced focal inflammation, we found elevated levels of neutrophils in infected joints. CCR2 and its ligand CCL2 are typically involved in the recruitment of neutrophils and inflammatory monocytes [22, 37]. In a model of antigen-induced arthritis, CCR2-deficient mice developed significantly reduced arthritis and neutrophil infiltration within their joints, while administration of WT neutrophils to CCR2−/− mice diminished protection [22]. However, similar to what others have reported for Borrelia burgdorferi–induced arthritis [38], we did not find a prominent role for CCR2 in musculoskeletal brucellosis.
CXCR2 is important in neutrophil recruitment and subsequent antimicrobial functions, including oxidative burst, degranulation, netosis, and phagocytosis, and is activated through the production of chemokines such as CXCL1 and CXCL2 [39, 40]. Levels of CXCL1 and CXCL2 in Brucella-infected IFN-γ−/− joints correlated with the onset of inflammation. CXCR2 deficiency did not enhance susceptibility to infection as B. melitensis loads in the spleens and joints of CXCR2−/− mice and control animals were similar. We also found that CXCR2−/− mice controlled Brucella infection as well as WT mice in the absence of anti-IFN-γ treatment (data not shown). Therefore, CXCR2, under these conditions, was not required for the clearance of Brucella. Others have demonstrated that, during chronic infection, neutrophils impede clearance of B. abortus, perhaps by reducing T-helper type 1 responses [41]. Thus, reduced neutrophil recruitment in CXCR2-deficient animals is not likely to result in enhanced susceptibility to Brucella infection.
Mice lacking CXCR2, in both our anti-IFN-γ–treated and IFN-γ−/− models, displayed markedly reduced symptoms of focal inflammation relative to control animals. These findings may be clinically relevant because elevated levels of CXCL8, a human chemokine that signals through CXCR2, were found in the synovial fluid of a patient with articular brucellosis [26]. CXCR2 was required for neutrophil recruitment to Brucella-infected joints. Studies using collagen-induced, K/BXN, and lipopolysaccharide-induced models of arthritis suggest that neutrophil depletion by anti-Gr1 antibodies is protective against disease [42, 43]. However, in experimental Lyme disease–associated arthritis, neutrophil depletion, with RB6–8C5 antibody, results in early induction of arthritis and infiltration of PMN/leukocyte-like cells that do not express Gr-1 [44]. Here, we found that CXCR2-deficient animals had reduced levels of CCL3 and TNF-α, compared with control animals. Some evidence suggests neutrophil recruitment can be dependent on CXCL2 inducing the release of CCL3 with subsequent induction of TNF-α [45]. Future studies will determine the role of neutrophils and the signaling pathways that recruit them in Brucella-induced focal inflammation.
The signaling pathways and cells that produce the CXCR2 ligands CXCL1 and CXCL2 have yet to be determined in our murine model, but it has been demonstrated that Toll-like receptor 2 is required for alveolar macrophages to produce CXCL1 in response to B. abortus infection [46]. In addition to professional phagocytes, cells native to the joint may also be producing CXCR2 ligands. One study showed that, when human fibroblast–like synoviocytes are infected with B. abortus, they produce IL-6, CCL2, and CXCL8 in a dose-dependent manner. Additionally, these supernatants recruited human PMNs and monocytes in vitro [47]. Furthermore, human osteoblasts upregulate their production of CCL2, IL-6, and CXCL8 upon coculture with supernatant from B. abortus–infected monocytes. In turn, medium conditioned by B. abortus–infected osteoblasts induced monocyte production of CXCL8, TNF-α, IL-6, and IL-1β [48].
Collectively, our study suggests blocking or inhibition of CXCR2 may be a potential therapy for treating Brucella-induced inflammation. Genetic ablation of CXCR2 resulted in reduced arthritis in a mouse model of Lyme disease [38, 49]. Similarly, blocking CXCR2 through various techniques has proven to be therapeutic in treating models of rheumatoid arthritis [50–52]. In addition, treating mice with allosteric inhibitors of CXCR1 and CXCR2 reduced the number of neutrophils in the synovium in a mouse model of antigen-induced arthritis [53]. Therefore, the targeting of CXCR2 or CXCR2 ligands may have potential as a complementary therapy, in combination with antibiotics, for treating osteoarticular complications of brucellosis.
Notes
Financial support. This work supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant 1R21AI119634 to J. A. S.) and the University of Missouri College of Veterinary Medicine and Research Board.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Presented in part: Brucellosis 2014 International Research Conference, Berlin, Germany, 9–12 September 2014; American Association of Immunologists Annual Meeting, New Orleans, Louisiana, 8–12 May 2015; Cold Spring Harbor Laboratory Microbial Pathogenesis and Host Response Meeting, Cold Spring Harbor, New York, 8–12 September 2015.